Stainless Steel Types
There are different types of stainless steel. For example, when nickel is added, the austenitic microstructure of the iron becomes stable. This crystal structure makes the steel non-magnetic and less brittle at low temperatures. The amount of carbon it contains is increased for higher hardness and strength. With heat treatments, these steels can be used in many products such as razors, knives and cutting edges. Manganese is also found in many steels in different proportions and helps to maintain the austenitic structure given by nickel, at lower costs.
Stainless steels are classified into five groups according to their crystal microstructure.
1. Austenitic Stainless Steels
2. Ferritic Stainless Steels
3. Duplex Stainless Steels
4. Martensitic Stainless Steels
5. Precipitation Hardened Stainless Steels (PH)
1. Austenitic Stainless Steels
The 300 series or austenitic stainless steels account for approximately 60% of the world's total stainless steel production. They contain a maximum of 0.15% carbon, a minimum of 16% chromium, and sufficient nickel and/or manganese to stabilize the austenitic structure from very low temperatures to the melting point.
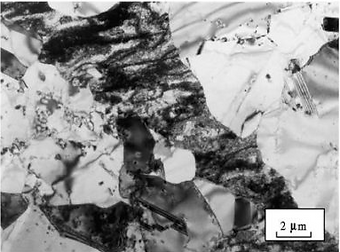
The most well-known type is 18/8 stainless steel (grade 304), which contains 18% chromium and 8% nickel. Steels such as AL-6XN and 254SMO, known as "superaustenitic" stainless steels, exhibit excellent resistance to chloride pitting and stress corrosion cracking thanks to their high molybdenum content (greater than 6%), added nitrogen, and high nickel levels.
The high alloy content of “superaustenitic” steels results in significantly higher costs. Therefore, it’s worth noting that although not identical, similar performance can also be achieved at a lower cost from ferritic or duplex stainless steel groups. The most widely known austenitic grades are 304 and 316.
Austenitic stainless steels are non-magnetic, cannot be heat treated, have high ductility, can be hardened by cold working, and offer excellent corrosion resistance, workability, and weldability. Their structure is FCC (face-centered cubic).
2. Ferritic Stainless Steels
Ferritic stainless steels typically do not contain nickel and instead include high levels of chromium (ranging from 10.5% to 30%) along with alloying elements such as molybdenum, titanium, and vanadium, which form carbides and stabilize the ferritic structure.
Their high chromium content generally provides excellent corrosion resistance. Ferritic stainless steels possess mechanical and physical properties similar to their close relatives, carbon steels. Unlike austenitic steels, ferritic ones are magnetic, cannot be heat treated due to their low carbon content, and can be easily cold rolled. The only applicable heat treatment for these steels is annealing.
Recently, extreme price increases and fluctuations in alloying elements—especially nickel—have accelerated the development of ferritic steels. As a result, new, cost-effective ferritic grades have been developed, offering corrosion resistance comparable to that of austenitic steels, with a broader range of applications and significantly lower cost.
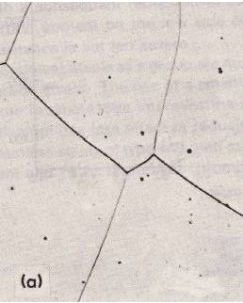
Here is the microstructure of a ferritic stainless steel containing 18% chromium and 0.03% carbon. It was rapidly cooled from 1150 ºC. (500x)

Here you can see a ferritic microstructure containing 12% Chromium. There are also small carbide particles. The material is an annealed material. (500x)
3. Duplex Stainless Steels
These steels typically contain roughly equal proportions of ferrite and austenite in their microstructures, and their corrosion resistance performance varies depending on their alloy content.
Compared to austenitic stainless steels, duplex grades offer higher strength and notably superior resistance to localized corrosion—particularly pitting, cracking, and stress corrosion. Their strength is largely attributed to high chromium content (19–28%), up to 5% molybdenum, and lower nickel levels compared to austenitic steels.
One of the most significant limitations of duplex stainless steels is their tendency to become brittle at very high and very low temperatures. If exposed—even briefly—to temperatures above 300 °C or below −50 °C, these steels can become brittle, necessitating re-annealing. The most widely recognized duplex stainless steel grade is 2205. Structurally, the ferritic portions exhibit a BCC (body-centered cubic) crystal structure, while the austenitic portions have an FCC (face-centered cubic) structure.


4. Martensitic Stainless Steels
However, due to the additional carbon it contains, it can be hardened and its strength increased by heat treatment like carbon steels. The basic alloying elements are: 12% to 15% chromium, 0.2% to 1.0% molybdenum and 0.1% to 1.2% carbon. It does not contain nickel except for a few martensitic grades.

Martensitic stainless steels, as seen in the microstructure example below, are magnetic. As carbon content increases, hardness and strength improve, while toughness and ductility decrease. Due to their high carbon content and the presence of other alloying elements, they can be hardened through heat treatment up to 60 HRC. After stress-relief through tempering (also known as temper heat treatment), optimal corrosion resistance is achieved.
Compared to ferritic and austenitic grades, martensitic stainless steels offer slightly lower corrosion resistance. However, they provide good machinability and formability. Depending on the type and proportion of alloying elements used, small amounts of retained austenite may be present in their structure.
Martensitic steels can be used very effectively in applications where strength and resistance to mechanical wear are required alongside corrosion resistance. They are also used as tool steels and have a wide range of applications. Their structure is BCT (body-centered tetragonal).
With a structure similar to ferritic steels, martensitic stainless steels resemble low-alloy, high-strength steels or carbon steels.
5. Precipitation-Hardened Stainless Steels (PH)
Also known as “age-hardened stainless steels,” precipitation-hardened stainless steels typically contain chromium and nickel. They lie between martensitic and austenitic grades, combining the advantageous properties of both.
Like martensitic grades, they can achieve high strength through heat treatment, while also offering corrosion resistance comparable to austenitic steels. Hardening is achieved by adding one or more alloying elements such as copper, aluminum, titanium, niobium, and molybdenum. The most commonly known grade in this group is 17-4 PH, also referred to as grade 630. Named for its composition of 17% chromium and 4% nickel, it also contains 4% copper and 0.3% niobium.
One advantage of precipitation-hardened stainless steels is that they can be supplied in a “solution-treated” condition, making them ready for machining or fabrication. After processing, a simple low-temperature heat treatment can be applied to increase strength as desired. Since the treatment is performed at low temperatures, it minimizes distortion or deformation in the finished material.
PH stainless steels are divided into three subgroups: Martensitic PH, Semi-Austenitic PH, and Austenitic PH. Depending on alloy content, PH steels can offer corrosion resistance comparable to austenitic grade 304. However, in their annealed (untreated) condition, corrosion resistance is quite low, so they should not be used before heat treatment. Their crystal structure may be BCT, FCC, or a combination of both, depending on the subgroup.

Here, the tempered martensitic structure of the 17-4PH grade was solution treated at 1040 ºC, air cooled, and aged for 4 hours at 495 ºC and air cooled. (1000x)

17-7PH Grade Stainless Steel
In this specimen, which is 17-7PH grade stainless steel, the material was heated at 760 °C for 1.5 hours, then air-cooled to 15 °C, held for 1.5 hours, and finally aged at 570 °C for 1.5 hours. At 1000x magnification, chromium carbide and ferrite particles can be observed within the martensitic matrix.
304 Grade Stainless Steel
ASTM 304 (304 Grade) is arguably the greatest success story in stainless steel. It accounts for 50% of all stainless steel production and roughly half of total stainless steel consumption, being used in nearly every industrial application.
304 is not just a stainless steel that fits every application; understanding its attributes provides a practical benchmark for assessing the suitability of stainless steel in a given application and serves as an excellent standard for comparing the materials within the austenitic stainless steel family. We all have experience with 304’s outstanding performance and excellent deep-drawing properties. Your cutlery (refer to the 18/10 and 18/8 markings), pressure cookers, sinks, and even the metallic parts in floppy disks are made from 304 stainless steel.
Composition:
-
Grade 304L (see Table 1) is a low-carbon variant of 304. It is occasionally used in welded assemblies to prevent potential corrosion sensitivity.
-
Grade 304H (see Table 1) increases strength—especially at temperatures above 500 °C—by containing a higher amount of carbon compared to 304L. However, this grade is not used in applications where there is a high risk of sensitive corrosion.
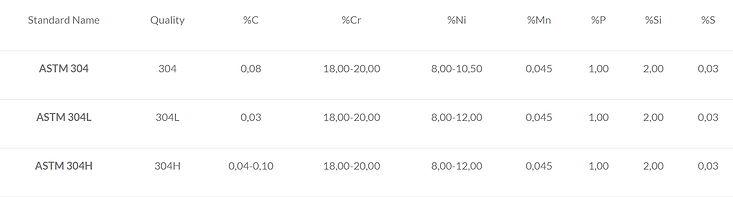
Note 1: Percentage values that are not given as ranges represent maximum values. Note 2: These values are defined in ASTM A240 for plates, sheets, and coils. Definitions for some other products may differ slightly from these values.
Both 304L and 304H are suitable for plates (flat products) and pipes, although 304H is less commonly found in stock. In some cases, 304L and 304H are stocked as standard 304 (with test certificates verifying whether the material is “L” or “H”).
Corrosion Resistance:
304 Grade stainless steel offers excellent corrosion resistance in a very wide range of applications. It does not rust in many architectural and construction applications. In addition, it is easy to clean and is resistant to organic chemicals, a broad spectrum of inorganic chemicals, and colored coatings—making it ideal for environments associated with food production and processing.
However, 304 Grade is susceptible to stress corrosion, pitting, and crevice corrosion in chloride environments at moderate temperatures—especially at temperatures above 50 °C when tensile forces are applied. On the other hand, when it is exposed intermittently to lukewarm chloride environments with regular cleaning (for example, in cookware and certain yacht fittings), it performs successfully.
Thermal Resistance:
304 Grade stainless steel exhibits excellent oxidation resistance in environments where it operates intermittently at 870 °C and continuously at 925 °C. However, if 304 is used in the 425–860 °C range and is followed by applications in aqueous environments at room temperature, its use is not recommended. In environments where the temperature fluctuates above or below this range, it may still sometimes perform well.
304L, on the other hand, is more resistant to carbide precipitation and can be used within the aforementioned temperature range. In applications where the material must maintain strength at elevated temperatures, higher carbon levels are required. For example, the AS1210 pressure vessel code limits the service temperature of 304L to 425 °C, while the use of standard 304 above 550 °C is restricted to carbon levels of 0.04% or higher.
Furthermore, 304 Grade exhibits excellent toughness at the low temperatures typical of liquefied gases and is consequently used in such applications.
Mechanical and Physical Properties:
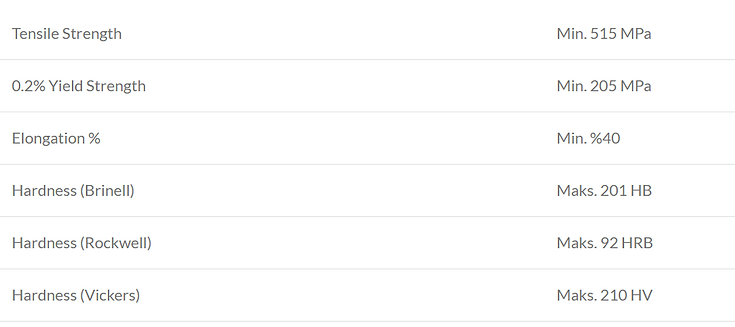

Magnetic Properties
Like other austenitic grades, solution-annealed 304 stainless steel is nearly non-magnetic (exhibiting very low magnetic properties). However, once cold-rolled, it can become significantly magnetic—though this magnetic effect can be reversed by annealing.
As with other austenitic steels, 304 can only be strengthened through cold working. Tensile strengths exceeding 1,000 MPa can be achieved, and special cold-worked orders with high strength can be produced according to the required product form and quantity (see ASTM A666 or EN 10088-2).
Annealing is the primary heat treatment applied to 304 grade. The process involves heating the material to between 1,010 °C and 1,120 °C, followed by rapid cooling—typically through water quenching.
Manufacturability
304 stainless steel exhibits excellent formability. It can be utilized in deep drawing operations without the need for an intermediate softening heat treatment. This outstanding attribute makes it a preferred material in the manufacture of items such as containers and cookware produced by deep drawing (commonly referred to as “sıvama”). In addition, it can be easily cut and formed into a variety of components used in industrial, architectural, and transportation applications.
304 is also highly weldable and compatible with all standard welding techniques (although oxyacetylene welding is generally not used). While proper post-weld cleaning is recommended, post-weld annealing is sometimes unnecessary for maintaining 304’s corrosion resistance. In contrast, 304L does not require post-weld annealing and is widely used in high-volume production.
The machinability of 304 is lower than that of many carbon steels. Standard austenitic steels like 304 can be machined effectively at slow speeds, with heavy feeds, using rigid, sharp cutting tools and appropriate cooling fluids.
Price Comparison
While an “initial cost” comparison alone is not adequate, the guide shown in Table 4 recommends using standard cold-rolled surfaces of sheet materials for building projects. In many applications, “lifecycle cost” parameters considerably enhance the attractiveness of stainless steel compared to competitors evaluated solely on initial cost.

Suitable Forms
316 grades are available in virtually every stainless steel product form, including coils, sheets, plates, strips, tubes, pipes, fasteners, rods, bars, angle products, wires, castings, and other shapes. Furthermore, 316 stainless steel is produced in a full range of surface finishes—from standard to custom textures.
Applications
The typical applications of 316 stainless steel can be summarized as follows: it is used in yacht fittings and structural components, particularly in maritime architectural structures, in harsh or industrial environments, in equipment for food and beverage processing, in hot water systems, as well as in chemical, petrochemical, mineral processing, photographic, and various other industrial applications.
Often described as “marine grade,” 316 is considered a level above 304 base quality.
Alternatives
In certain applications and service environments, alternative grades to 316 should be considered:
-
Strong Reducing Acid Environments: Consider alternatives such as 904L, 2205, or super duplex grades.
-
Environments Above 50–60 °C with Chloride Content: Opt for grades like 2205, super duplex, or super austenitic steels that offer superior resistance to stress corrosion cracking and pitting.
-
High Demands Such as Heavy Welding (316L), Additional Processing (an enhanced form of 316 with improved machinability), or Requirements for High Strength or Hardness (martensitic or precipitation-hardened grades).
Low-Nickel Austenitic Stainless Steels
304 and 316 grades—popular for their excellent corrosion resistance (stemming from their austenitic microstructure), combined with favorable mechanical, physical, and manufacturing properties—are among the most recognized stainless steels. The austenitic structure is formed with an approximate addition of 8–10% nickel. However, nickel is not the sole element responsible for creating the austenitic structure; manganese, nitrogen, carbon, and copper are also employed for this purpose.
Cost of Nickel and Its Addition to Stainless Steel
Generally, the cost of stainless steel is determined by the prices of its alloying components. Although chromium—the primary element responsible for forming the protective chromium oxide layer that prevents corrosion—is not expensive, the addition of elements that enhance corrosion resistance (especially molybdenum) or improve manufacturability (particularly nickel) significantly increases the cost. For example, nickel’s price was approximately $5,000–$6,000 per ton in 2001, but by the end of May 2007, it had risen to $50,000 per ton. Similarly, the price of molybdenum increased from about $8,000 per ton in 2001 to approximately $40,000 per ton today.
These cost increases have directly impacted the following two grades: 304 (18% Cr, 8% Ni) and 316 (18% Cr, 10% Ni, 2% Mo)—with 316 being the most affected. Additionally, other stainless steels with high alloying content such as the 2205 duplex grade (22% Cr, 5% Ni, 3% Mo) have also been influenced. The role of the alloying elements is primarily to modify the microstructure in ways that affect corrosion resistance as well as the mechanical and manufacturing properties. One widely used approach to quantify corrosion resistance is the Pitting Resistance Equivalent (PRE) ratio, defined as:
PRE = %Cr + 3.3×%Mo + 16×%N
The PRE ratio is used to rank materials based on their resistance to pitting corrosion, although it does not account for every factor influencing overall corrosion behavior. As seen, in addition to chromium, both molybdenum and nitrogen play significant roles in providing resistance to this type of corrosion.
The Role of Nitrogen Relative to Molybdenum and Nickel
Although nitrogen is much less expensive than molybdenum and nickel, its solubility in steel is limited to approximately 0.2%, which in turn limits its impact on corrosion resistance. The steel’s microstructure is governed by the balance between ferrite-forming and austenite-forming elements. In forming the austenitic structure, elements such as carbon, manganese, nitrogen, and copper serve as viable alternatives to nickel. All these elements are less costly than nickel. As observed in the PRE formulation, each element influences corrosion resistance differently, and it is not feasible to completely eliminate nickel from austenitic grades.
Even though manganese is not as potent as nickel, it is a key stabilizer of the austenitic structure. In fact, Cr-Mn steels exhibit a higher degree of cold-work hardening compared to Cr-Ni steels. While the PRE equation does not explicitly account for manganese, nickel tends to have a more beneficial effect in environments where corrosion aggression is more severe. Moreover, there is a synergistic relationship among alloying elements: nitrogen significantly stabilizes the austenitic structure, and as manganese aids in stabilization, it concurrently enhances the solubility of nitrogen in the steel.
The Rise of 200 Series Stainless Steels
Manganese has evolved into an important alternative to nickel—from a minor additive to a major constituent. The development of high-manganese austenitic steels began roughly 60 years ago, during periods of sharply rising nickel prices. In those times, Cr-Mn-Ni grades such as 201 (17% Cr, 4% Ni, 6% Mn) and 202 (18% Cr, 4% Ni, 8% Mn) emerged as alternatives to traditional Cr-Ni grades like 301 and 302 under the AISI classification, and they continue to be produced and utilized today. However, their consumption has declined in recent times compared to their Cr-Ni counterparts. The lower demand for these grades can be attributed to several factors:
-
High Cold-Work Hardening: Although this may be an advantage in some applications, it can also be a drawback in others.
-
Inferior Surface Quality: Their surface finish is significantly lower, making them less suitable for certain applications.
-
Additional Production Costs: Increased production expenses and high refractory wear during melting.
-
Reduced Corrosion Resistance: In some working environments, their corrosion resistance is not as high as that of Cr-Ni grades.
Additionally, it is noteworthy that while Cr-Ni and Cr-Mn-Ni austenitic grades are inherently non-magnetic, scrap metal dealers typically determine the scrap value based on the approximate nickel content.
Recent Developments
Recently, we have witnessed significant progress in Cr-Mn-Ni austenitic grades. The most notable development is occurring in India, where their use in kitchenware and cooking equipment is on the rise. The suitability of low‑nickel, high‑manganese grades for high cold‐work hardening is acceptable for these applications, and copper additions are employed to mitigate this issue.
Due to local economic factors, India is achieving favorable results in both development and production for these applications. Likewise, other Asian countries have built a robust market for these grades and have ramped up production in recent times. In particular, production and usage of these grades are growing daily in the Chinese market.
They are also manufactured in countries such as Taiwan, Brazil, and Japan—with nickel contents ranging from 1% to 4% and manganese contents reaching up to 9%. None of these grades have yet been incorporated into ASTM or other international standards. The production of low‑nickel austenitic grades is increasing very rapidly. According to ISSF data from 2003, production of these grades reached 1.5 million tons, representing 7.5% of the world’s total stainless steel production. In 2004, they were estimated to account for about 25% of stainless steel production in China. Given the soaring nickel prices since 2006, their production rate is believed to be even higher today.
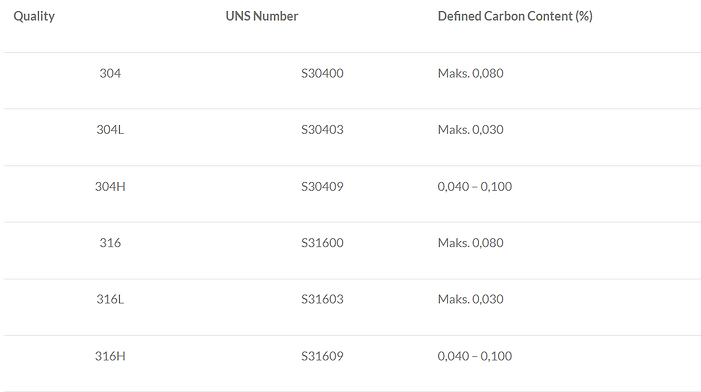
Carbon Content
General austenitic stainless steels such as 304 and 316 are produced with controlled low and high carbon contents, designated as the “L” and “H” grades, respectively. Low‑carbon or “L” grades are used to reduce (or prevent) sensitization and corrosion at high temperatures. The critical temperature range—where welding or specific applications are problematic—is between 450 °C and 850 °C. “L” grades are typically found in thick flat products (usually above 5 mm in thickness).
High‑carbon “H” grades are utilized in applications requiring higher strength. The interchangeability of “L” and “H” grades is frequently encountered in practice.
What Are “L” Grades and Where Are They Used?
“L” grades are employed in applications involving high temperatures, including scenarios where the welding of medium to large-sized materials is performed. Low carbon content helps prevent or delay intergranular carbide precipitation (commonly referred to as “sensitization”) in corrosive environments. There is an incubation period for carbide precipitation within the 450–850 °C range, and because the time for carbide formation is largely dependent on carbon content, a lower carbon level enhances resistance to this problem. Owing to their properties, “L” grades are typically available in the form of plates, sheets, pipes, and often round bars. When high‑temperature exposure or heavy welding is not required, “L” grades are generally interchangeable with standard grades.
What Are “H” Grades and Where Are They Used?
“H” grades are the high‑carbon versions of standard grades and offer enhanced strength—especially at elevated temperatures (typically above 500 °C). They exhibit high creep strength under long‑term loading conditions and are primarily produced in the form of plates and pipes. In addition to the common 304H and 316H grades, high‑carbon versions of grades 309, 310, 321, 347, and 348—as defined in ASTM A240/A240M—are also available. However, if maintained within the 450–850 °C temperature range, these grades are highly susceptible to carbide precipitation (referred to as “sensitization”). Should sensitization occur, their room‑temperature ductility and toughness decrease significantly, and their corrosion resistance is markedly compromised.
What Is the Difference?
The chemical compositions of 304 and 304L are identical aside from their carbon content. In theory, 304L permits up to a maximum of 12% nickel, compared to a maximum of 10.5% nickel in standard 304. However, due to high nickel prices, both grades are typically produced with a minimum nickel content of about 8.5%. Neither grade has a defined minimum carbon limit; a material with 0.02% carbon content satisfies the requirements for both 304 and 304L.
For 304H, the carbon content is limited to between 0.04% and 0.10% (note that no minimum is specified), and unlike standard and “L” grades, 304H does not have a maximum nitrogen limit of 0.1%. Otherwise, its chemical composition is the same as that of standard 304. Additionally, all austenitic “H” grades must possess an ASTM grain size of No.7 or larger.
The relationship among 316, 316L, and 316H is analogous to that of the 304 series—restrictions apply only regarding carbon content, nitrogen content, and grain size. (Refer to Table 1 for the carbon content of grades as specified in ASTM A240/A240M.) In certain product specifications, such as those for pipes and tubes, the maximum carbon content for 304L and 316L is limited to 0.035% or 0.040%, while all other specification parameters remain the same.
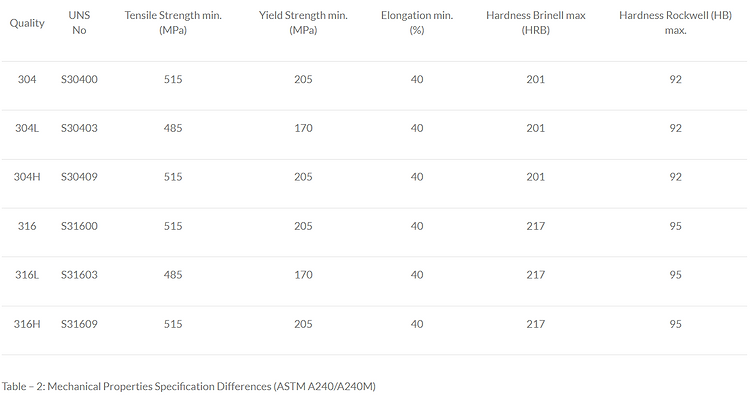
-
Table 2 presents the specifications of the mechanical properties as defined by ASTM A240/A240M. In practice, steel producers typically ensure that their production of “L” grade materials also meets the requirements of standard grades. For example, the tensile and yield strengths of all 304L grades exceed 205 and 515 MPa, respectively. This approach enables the production of materials that cater to a broad market by fulfilling both standard and “L” grade quality requirements.
5. The dimensional and other requirements for Standard, “L”, and “H” grades are identical.
6. Pressure vessel and pipe pressure specifications impose limits on the operable pressure range at high temperatures for each grade.
These specifications do not allow the use of “L” grades at temperatures above 425 °C. Furthermore, for temperatures exceeding 550 °C, a minimum carbon content of 0.040% is required. Consequently, regardless of the “L” designation, 304 and 316 grades containing only 0.020% carbon are not permitted for use in such applications. Standard and “L” grades may be used between room temperature and the maximum temperature allowed for “L” grades provided their chemical composition and mechanical properties meet the specified requirements.
Pressure vessel specifications permit the use of “H” grades together with standard grades, as long as the required strength values are met.
Alternative Grade Usage
In situations dictated by application requirements, Standard, “L”, and “H” grades may be substituted for one another. This interchangeability is subject to the following conditions:
-
“L” grades can replace standard grades in applications that do not require high-temperature strength, provided that their mechanical properties satisfy the necessary requirements. In general, “L” grades typically meet the requirements of standard grades. However, each property must be rigorously verified via the manufacturer’s certificate to ensure compliance. It is common practice for manufacturers to produce or supply “L” grade material in response to standard grade orders. In practice, as long as the material is used in accordance with the applicable specifications and no issues arise between the component manufacturer and the end user, no problems should occur.
-
Standard grades may be used as “L” grades provided their carbon content complies with the carbon content limitations specified for “L” grades.
-
The increasing use of double-certified products—particularly in plates, sheets, pipes, and bars—is becoming a common practice. These materials fully comply with 304/304L or 316/316L grades. While the use of double-certified products is applicable for “L” grades, this practice does not extend to “H” grades. If an application requires “H” grade material, this must be explicitly specified at the ordering stage. Standard grades may also be used in place of “H” grades provided that their carbon content meets the “H” grade requirements. Any additional discrepancies in microstructure grain size may be addressed through supplementary inspections. The material and its certification should be designated as “standard”; otherwise, the material is produced as “H” grade. The details on the test certificate will reflect the grade’s specific requirements.
-
“H” grades may be used as standard grades as long as their carbon content does not exceed 0.080% and their nitrogen content is a maximum of 0.10%. Although they typically meet these criteria, their certification should still be verified.

